Bond Builders: Top Tips for Product Development & Must-Knows for Claim Support
- TRI Princeton

- Nov 24, 2024
- 7 min read
Updated: Nov 26, 2024

Dr Paul Cornwell, Director at TRI Princeton

Dr Philippa Cranwell, Technical Content Creator at TRI Princeton
At TRI we are often asked to provide claims support for bond builder products. This extended blog post will look at what bond builders are and how they work, the three main types, and finally give examples with a brief overview of the chemistry behind them.
Introduction
Hair damage is big business with damage control a particular concern for the modern consumer; a survey of 23,000 people by Dyson in 2021 revealed that 70% of people believed they had damaged hair with issues including dandruff, hair loss and going grey. One of the primary manifestations of hair damage is breakdown of the cuticle and cortex, which can lead to hair looking frizzy, dull and breaking easily. A recently developed way to address this is through the use of bond builders, a class of active ingredient that “…is able to penetrate into the hair and improve or restore the internal structure of hair, giving rise to an improvement in hair mechanical properties.” This approach, where the actives penetrate the hair fiber and rebuild hair from within, rather than just residing on the cuticle, is game-changing and projections from Data Bridge Market Research suggest that the hair bond multiplier market, i.e. development of products that sustain, create or enhance bonds in hair, will have a CAGR of 7.8% between 2021 and 2028.
First things first: peptides, proteins and their importance to this technology, and hair
Within science, peptides and proteins are in the spotlight. Where peptides are small, low molecular-weight compounds usually containing between 2 and 50 amino acids, proteins are much larger, sometimes containing hundreds of amino acids. Both play significant roles within the body, with functions including cell signaling, providing structure and transportation.
While the use of peptides as therapeutic agents to treat diseases such as diabetes or obesity has been headline news with the recent invention of Ozempic and Wegovy, peptides are also having impact through their use as actives within cosmetic science and personal care. Common claims usually relate to their ability to reduce fine lines, combat acne, and repair skin from UV damage. For proteins, their larger cousins, when used as an active there are two options: they can either be incorporated into treatments or be the target of a particular medicine or cosmetic agent. For example, the protein collagen is commonly included in face creams to help combat skin sagging and restore a youthful glow, however collagen can also be the target of other actives such as retinol (vitamin A) or palmitoyl tripeptide-38 (Matrixyl Synthe'6), which are both known to promote collagen synthesis.
Bond builders for hair are also designed to target proteins. In this case, the proteins in question are within the hair cortex, which provides the bulk of hair mass and gives hair its tensile strength. There are two key protein-containing structures within the hair cortex: the intermediate filaments, which are made from keratin proteins and provide strength, and the matrix, which binds everything together, contains keratin-associated proteins and gives hair its flexibility. Bond builders repair any breaks in these protein structures, for example through chemical bleaching or mechanical stress, restoring hair strength and improving hair health.

Figure 1: The structure of hair showing how amino acids assemble into keratin proteins, and how these proteins then assemble into intermediate filaments and macro-fibrils, giving hair it’s tensile strength.
Classes of bond-builder
There are currently three classes of bond builder: organic acids, proteins and peptides, and lipids. In all cases, the active ingredients penetrate the hair structure and chemically alter it. We’ll look the three types in further detail below and discuss the chemistry behind them in a later section.
Organic acids
Of the three types of bond builders, the most widely researched are those that are classified as acids and includes compounds such as malic acid, maleic acid, succinic acid, citric acid, calcium lactate, hydroxypropyl gluconamide and glycine-betaine (from sugar beet molasses). It has been shown that treating hair with malic acid reduces the water content, which has been proven to improve hair set durability and style hold. In addition, voids inside chemically damaged hair can be closed, which enhances light transmission and color vibrancy.
Proteins and peptides
Low molecular weight proteins and peptides are well-studied and have been shown to penetrate into hair through fluorescence microscopy. Interestingly, penetration into hair by proteins and peptides is better when hair is damaged due to the porosity and more permeable cell membrane complex. In analogy with organic acids, peptides and proteins also affect the hair’s water content, as well as induce changes to the tensile properties.
However, the molecular weight of the protein is key. High molecular weight proteins are less able to penetrate hair, presumably due to their size, but can be delivered to the hair surface where they reduce premature fractures and to increase break stress, possibly through a bulk adhesive effect. Low molecular weight proteins can enter the hair structure and can interact with the native amino acids.
Lipids
Lipid bond builders exhibit very strong hydrophobic interactions with native lipids in the cellular membrane complex (CMC). Penetration of these materials can be quantified and visualized, with an increase in fatigue strength often observed when successful.
How do they work?
Regardless of type, bond builders are designed to interact with structures inside the hair fiber, either proteins or peptides, or lipids. The proposed mechanisms are different for each type of builder. The two case studies we’ll consider are organic acids and peptides, with information and data taken from work by Förster and et al in 2018.
Organic acids: how they work
Olaplex is well-known product and fits into the ‘organic acid’ category of bond builders. In terms of structure, there are two terminal maleic acid head groups and a central glycol linker. Joining the linker with the head groups are ionic bonds between the carboxylate salt and the protonated primary amine, Figure 2a. There are several theories relating to how organic acids more generally interact with the hair, which will be outlined below.
The first theory is that the acids enter the keratin fibers and form cross-links, Figure 2b. This relies upon ionic (salt) interactions between proteins in the keratin back-bone and the active acid species.
The second option is that a covalent bond is formed between a chemical component of the active (a Michael acceptor) and a thiol residue in a cysteine in the keratin backbone, Figure 2c. This mode of bonding is proposed in the patent for Olaplex and is similar to binding mode of proteins to drug molecules in the body but there are questions around whether this is likely under the oxidizing conditions required for hair bleaching or coloring, as the cysteine thiol (SH) residues are likely the be rapidly oxidized to the sulfenic, sulfinic or sulfonic acid, which will render them less nucleophilic.
The third option is that the organic acid itself can undergo polymerization during hair treatment. This has been shown to occur with maleic acid in the presence of potassium persulfate (KPS) and polyvinylpyrrolidone (PVP; a common hair product additive), with KPS present in high lift lightening treatments, Figure 2d.
The final hypothesis is that chelation of metal ions in the hair from everyday environmental insults could be achieved through the organic acid, Figure 2e. This chelation would remove metal residues from the hair, e.g. Ca(II), and improve the hair fiber mechanical properties.
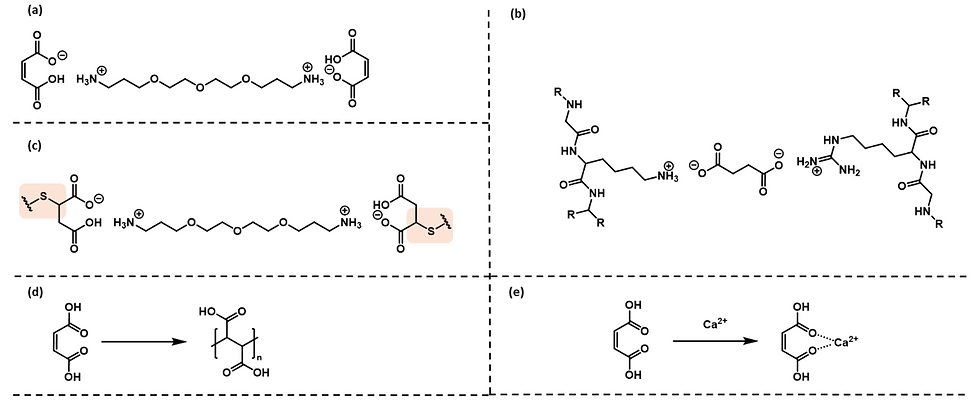
Figure 2: (a) Olaplex chemical structure; (b) dicarboxylic acid cross-linking two cationic amino acid side-chains; (c) covalent bond formed through Michael addition of SH groups in cysteine residues of keratin side-chains into maleic acid; (d) polymerization of maleic acid; (e) chelation of metal ions by acid residues in the active.
Proteins and peptides: how they work
As noted earlier, the key difference between proteins and peptides is their size, which also has an impact upon their mechanism of action. Large proteins, or proteins with high molecular weight, are unlikely to penetrate the hair fibers. However, they can penetrate cracks in damaged hair and form a film on the surface. Recent work at TRI suggests that this film can give adhesive effects, which can increase break stress and reduce premature fractures, Figure 3a.
Smaller proteins, or low molecular weight peptides, can penetrate the hair fibers more easily. Recent work at TRI suggests that proteins can increase hair fiber diameter leading to plasticization of the hair, Figure 3b.

Figure 3: (a) High MW proteins penetrate cracks in damaged hair and, through their film-like and adhesive effects, increase break stress and reduce premature fractures; (b) Low MW proteins can penetrate between matrix proteins in damaged hair and increase fiber diameter.
Claims testing: how TRI can help
When it comes to claims testing for bond-builders, there are three areas that should be addressed:
That the active is penetrating the hair shaft;
That the active changes the material properties of the hair;
That the active affects the bonding present in the hair.
To prove that the active is penetrating the hair shaft, a few options are available. Infrared (IR) and confocal Raman spectroscopy are the simplest to execute, as they require minimal sample preparation and can be completed fairly quickly. However, they do require the active to have functional groups that are compatible with those spectroscopic techniques, which is not always the case. An alternative is fluorescence microscopy, where a tagged version of the active ingredient can be tracked as it penetrates into the hair sample. The final option is ToF-SIMS, where mass spectrometry can be used to trace materials that do not have distinct IR or Raman absorption bands, and does not require the attachment of any markers or tags.
For changes to the material properties of the hair effected by bond building, the most relevant are in relation to hair strength therefore fatigue and tensile testing are often recommended. Fatigue testing recreates the conditions experienced by hair fibers during grooming, with the fatigue tester repeatedly applying small deformations until the hair fiber breaks. Tensile testing involves extending hair fibers once, until they break, and can be completed under both wet and dry conditions.
Finally, testing that considers if additional bonds have been formed in the hair are possible. Of these, differential scanning calorimetry (DSC) provides insight into levels of protein damage and repair after a treatment, whereas confocal Raman and IR can reveal new S–S and hydrogen-bonds, respectively. Other techniques are possible, such as X-ray crystallography and NMR, but require specialist equipment and expertise. Contrary to popular belief, scanning electron microscopy (SEM) cannot prove that a bond-builder has strengthened hair!
With all of these tests, however, the key is ensuring that the experiments are comparable, and the variables are controlled. TRI Princeton can help you design experiments and ensure that you results form a good basis for any bond building claims.
Contact us today to discuss your hair and scalp product testing needs.


